Botox Fda Label 2025. Botox cosmetic (onabotulinumtoxina) is indicated in adult patients for the temporary improvement in the appearance of: However, this new approval provides a more.

This expanded botox ® dosing guidance provides physicians the ability to treat based on clinical assessment of a patient’s spasticity and anatomy while staying within the. Food and drug administration (fda) has approved a.
Botox FDA prescribing information, side effects and uses, • moderate to severe glabellar lines associated with corrugator and/or procerus muscle activity
Botox FDA prescribing information, side effects and uses, The following document was submitted to the fda by the labeler of this product guangzhou yanxi biotechnology co., ltd.
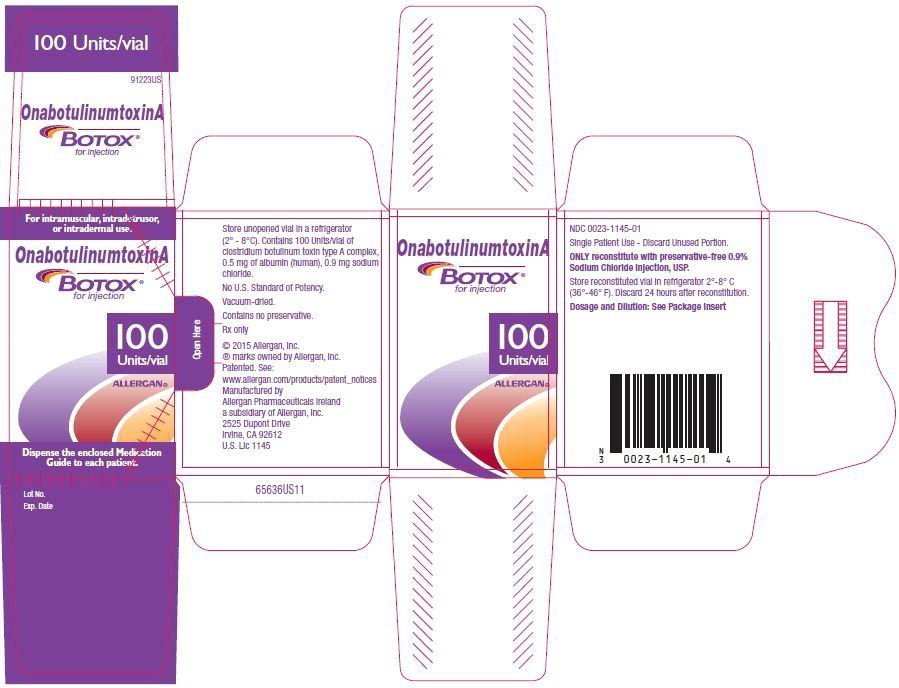
Botox Cosmetic FDA prescribing information, side effects and uses, Fda approval of botox ® cosmetic for temporary improvement in the appearance of moderate to.
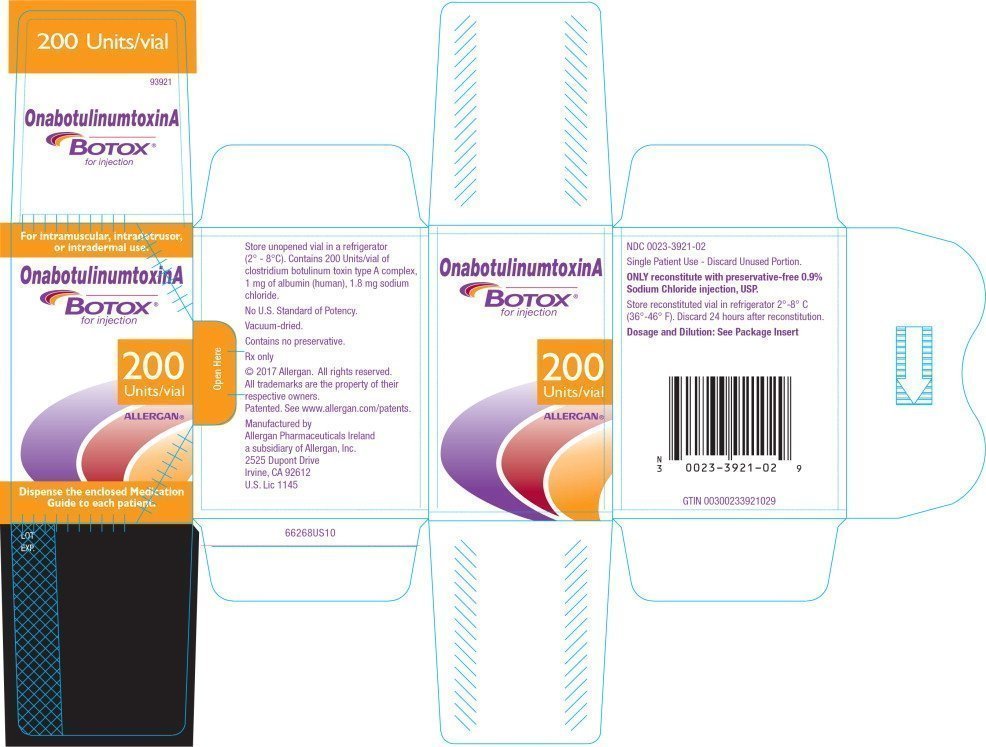
FDA Label for Botox Injection, Powder, Lyophilized, For Solution Intradermal; Intramuscular, Fda approval of botox ® cosmetic for temporary improvement in the appearance of moderate to.
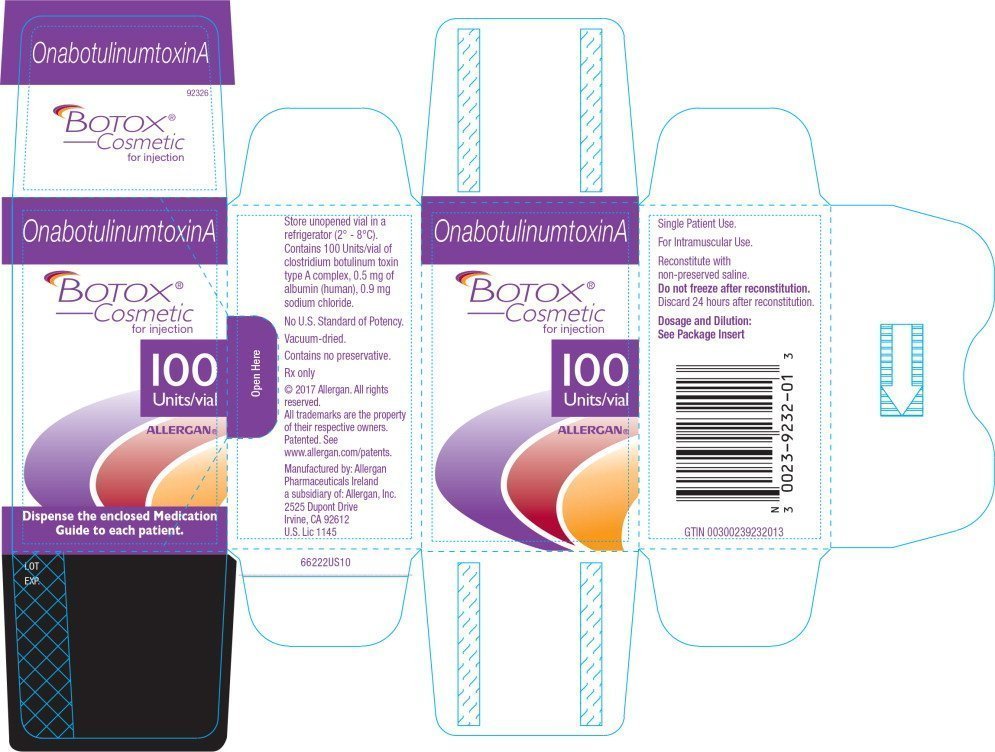
BOTOX Cosmetic celebrates 20 years since first U.S. FDA approval PharmaLive, However, this new approval provides a more.

FDA Approved BotulinumA Products Empire Medical Training, The us food and drug administration (fda) has approved a label expansion of onabotulinumtoxina (botox) to include 8 new muscles in the treatment of upper limb spasticity.

FDA Approves Expanded BOTOX® (onabotulinumtoxinA) Label to Include Eight New Muscles to Treat, Abbv) company, today announced that the u.s.
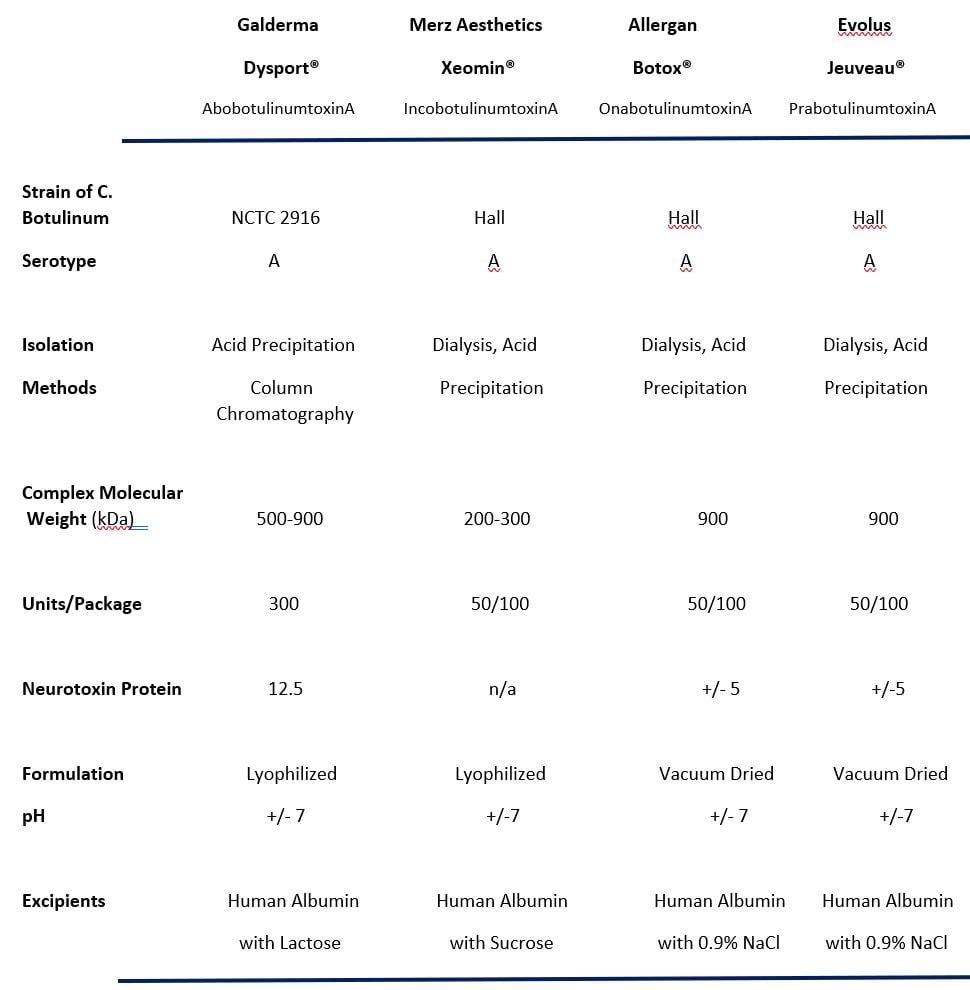
Fda orders warning label for botox and similar drugs, Fda approval history for botox cosmetic (onabotulinumtoxina) used to treat facial wrinkles, glabellar lines, platysma bands.

August September Back To Business Botox, The following document was submitted to the fda by the labeler of this product guangzhou yanxi biotechnology co., ltd.

Top Off Label Uses For Botox OVME Explains YouTube, Fda approval of botox ® cosmetic for temporary improvement in the appearance of moderate to.
